Canada has a list of industrial chemicals that it considers "toxic" [
Link to gov't site]. It has been announced that the list has been just increased by 11 more. It is somewhat alarming that some of the chemicals listed as cosmetics additives or additives to plastics that are in your house right now. Actually the whole cosmetics industry has not covered itself with glory from the get-go. I mean the reality of the cosmetics industry is two-fold: 1) to give a false impression and 2) to achieve goal #1 at any and all cost. There are people out there who would gladly trade a shorter life for "looking good". The illusion of a healthy glow has been achieved for centuries by coating the face with compounds of mercury or antimony and 11 doctors out of 10 will tell you that is a bad bad idea. Indeed, the plastics industry has always tweaked the properties of the pure plastics with
additives to suit the end use and the attitude has always been that the additives have not been mobile once
Incorporated in the plastic. That would appear to have been an assumption of disputable validity.
The whole moving to a shack in the woods and living off the land is looking better all the time.
The Additions to the Toxic List
(Links to mostly Wikipedia (click on name))*
1]
Acetic acid ethenyl ester [Vinyl acetate] Linked to cancer. Used in products such as abrasives, fragrances, perfumes and deodorizers.

Now this is just unfair, it is a nice volatile compound with a pleasing odour and has served us well for centuries and now we turn our backs on it for causing cancer.
2]
1,3-Butadiene, 2-methyl [Isoprene] Linked to cancer. Used in rubber and plastic manufacturing.

But, but, but ... it's a NATURAL compound! Nature wouldn't hurt us would it? I mean just who is in charge here maybe we should just show Nature who's the boss. Yeah, that's what we will do. We will kick old Nature right where it hurts, we will pollute the ground, water and air and spit in Nature's eye and tell it to do its best. I mean really what can it do if we drag chemicals out of all natural chemical contexts and use the chemicals in ways that Nature never intended?
3]
Thiourea Linked to cancer. Used in electronic products, mining, textiles, dry
cleaning and hair preparations and cosmetics.

I like this compound I like it
alot. I can think of chemical reactions I would like to do with this chemical if I had a nice dark lab and a warm retort (look it up kids it only sounds dirty).
4]
Oxirane Linked to cancer and persistent in the environment. Used in epoxy resins for paints, coatings, adhesives and other products, and to produce synthetic glycerin.

This has just gotta be the coolest compound on the toxic list. No organic modelling kit will allow you to build this simple molecule because three
membered rings are supposed to be rare and unstable and yet I can bet that you have driven down the road and have been passed by a tanker transport of this stuff. Cool but it will tear into organic compounds like a rabbit into wet sand.
5]
C.I. Pigment Yellow 34 [chromate yellow] Contains chromium and lead. Linked to cancer. Used as colorant in plastics, inks, paints, coatings, adhesives, textiles and sealants, artists' supplies, cars, vinyl packaging, toys.
I got nothing on this one I would have thought eliminating lead and chromium would have been taken care of years ago. I guess traditional use trumps risk. If you tried to bring something like this on the market now as a new compound you would never get it onto the North American market except in Thomas the Tank Engine toys.
8]
Cyclotetrasiloxane, octamethyl [D4]Deemed an environmental hazard. Used in construction, textiles, leather and hide tanning, paper products, plastic packaging, household appliances, computers, motor vehicle parts and cleaning compounds.
9]
Cyclohexasiloxane, dodecamethyl [D6] Deemed an environmental hazard. Used in cosmetics, beauty supplies, perfumes, personal care products, pharmaceuticals and drug products, paper bags and paper products, rubber products, medical equipment and supplies, cleaning compounds, polishes, foods, paints, coatings and adhesives. Building blocks of silicone, application includes breast implants.
10]
Cyclopentasiloxane, decamethyl [D5] Deemed an environmental hazard. Used in health and personal care products, footwear, automotive parts, construction, mining and oil/gas extraction, transportation, warehousing and storage, pharmaceuticals, toiletries and cosmetics. Building blocks of silicone, application includes breast implants.

This is one fancy family of environmentally hazardous chemicals. The cyclic
siloxanes have an amazing and
surprising chemistry and the fact that they went from lab to industry so fast is a measure of the niche that they occupy. Makes me wonder what they have to replace these guys. I got to say that this really makes me reconsider the pectoral and butt implants I was thinking about.
11]
Phenol, 2,4,6-tris [1,1-dimethylethyl][2,4,6-tritert-butylphenol]Deemed an environmental hazard. Used as a fuel additive.

OK, now just wait a minute, this is super
mesityl phenol. I mean how can you list a chemical with "super" in its name? Who is going to protect us now when we really need an organic oxidant?
* I am well aware that I have gone on record that Wikipedia is a bad source but it is late and I have been assimilated.



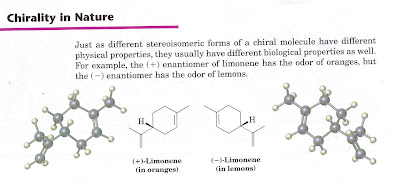
 In fact the only way we can separate handed molecules is with another handed molecule. The question of course is where the broken symmetry comes from but that is an issue for another day. Suffice it to say that chemists have a unique interest in any sort of process that is capable of sorting left and right handed objects.
In fact the only way we can separate handed molecules is with another handed molecule. The question of course is where the broken symmetry comes from but that is an issue for another day. Suffice it to say that chemists have a unique interest in any sort of process that is capable of sorting left and right handed objects.



 But, but, but ... it's a NATURAL compound! Nature wouldn't hurt us would it? I mean just who is in charge here maybe we should just show Nature who's the boss. Yeah, that's what we will do. We will kick old Nature right where it hurts, we will pollute the ground, water and air and spit in Nature's eye and tell it to do its best. I mean really what can it do if we drag chemicals out of all natural chemical contexts and use the chemicals in ways that Nature never intended?
But, but, but ... it's a NATURAL compound! Nature wouldn't hurt us would it? I mean just who is in charge here maybe we should just show Nature who's the boss. Yeah, that's what we will do. We will kick old Nature right where it hurts, we will pollute the ground, water and air and spit in Nature's eye and tell it to do its best. I mean really what can it do if we drag chemicals out of all natural chemical contexts and use the chemicals in ways that Nature never intended? I like this compound I like it
I like this compound I like it  This has just gotta be the coolest compound on the toxic list. No organic modelling kit will allow you to build this simple molecule because three
This has just gotta be the coolest compound on the toxic list. No organic modelling kit will allow you to build this simple molecule because three  This is one fancy family of environmentally hazardous chemicals. The cyclic
This is one fancy family of environmentally hazardous chemicals. The cyclic 


















 All in all I was very impressed. They are teaching about 1000 students in classes of about 120. We sat in on one regular lecture given by my post-doctoral supervisor. It was clear that the students had bought into the whole concept of the text and most were recording their notes directly into their textbooks.
All in all I was very impressed. They are teaching about 1000 students in classes of about 120. We sat in on one regular lecture given by my post-doctoral supervisor. It was clear that the students had bought into the whole concept of the text and most were recording their notes directly into their textbooks.