There are symmetry laws written into the basic code of the Universe that we live it. If indeed Nature abhors a vacuum it is positively fanatical about breaking symmetry. The more one reads about them the more one is amazed by how basic symmetry rules control so much of what we know about anything.
Simply put breaking symmetry in any fundamental kind of way is strictly forbidden. When symmetry is broken the consequences are pretty amazing. Indeed, if you read Singh's "Big Bang" it is argued that the Universe itself is the result of a symmetry breaking event.
That said, in my discipline of chemistry the most important symmetry rules are written into the nature of tetrahedral molecular substructures in a phenomenon we call chirality. In terms of geometry, if you have a molecule with any point in it where there are four unique molecular bonds to the same atom there are two distinct spatial arrangements that are possible. For simplicity we call them left handed and right handed. It just so happens that the proteins in our bodies are, in fact, long chains of substructures that all have these tetrahedral atoms (pretty much each one an amino acid). What is remarkable (and amazingly improbable) is that each one of these atoms is the left handed version.
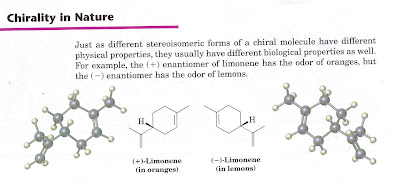
McMurry, 5th Ed.
Why is this amazing or improbable? The universal symmetry laws say that when molecules or atoms that are not left handed or right handed form a tetrahedral atom that there must be a 50:50 mix of left and right handed products. That is why scientists and chemists in particular are obsessed with Escher prints. Escher was an artist that made his money creating images where left and right handed images generated each other ...

In fact the only way we can separate handed molecules is with another handed molecule. The question of course is where the broken symmetry comes from but that is an issue for another day. Suffice it to say that chemists have a unique interest in any sort of process that is capable of sorting left and right handed objects.
Well a new one has surfaced.
It was in the Globe and Mail today that another right sneaker has washed up on a beach in British Columbia. What makes that odd is that the foot was still in it. What makes it odder still is that this is the fourth right foot sneaker with a foot still in it to wash up in recent days.
In the absence of a real twisted murderer loose on the West Coast it would appear that this is the result of a natural process of some kind where accidents or suicides result in bodies washing down river and out into the ocean where natural dismemberment results in the feet separating from the bodies but that the right handed foot / sneaker combination follow a different path than the left handed sneakers. The importance of the sneaker is to provide protection for the foot so it stays intact and the natural buoyancy of the sneaker makes the dismembered foot float.
All in all it would appear that a right handed object floating in the ocean is somehow polarized and separated from an equivalent left handed object. The proof of this theory would be to go back to the river source of the bodies and examine the coast in the opposite direction from where the right handed sneakers were found. If this theory is right there should be a stretch of BC coastline where the left footed sneakers accumulate. You heard it here first.
I'm just sayin'.




 In fact the only way we can separate handed molecules is with another handed molecule. The question of course is where the broken symmetry comes from but that is an issue for another day. Suffice it to say that chemists have a unique interest in any sort of process that is capable of sorting left and right handed objects.
In fact the only way we can separate handed molecules is with another handed molecule. The question of course is where the broken symmetry comes from but that is an issue for another day. Suffice it to say that chemists have a unique interest in any sort of process that is capable of sorting left and right handed objects.